Life Science News: New Psoriasis Treatment Breakthrough
Psoriasis is a skin disorder that causes skin cells to multiply faster than normal. Currently, there is no cure for psoriasis, but there are methods for treating it. In this article, we’ll dive into available treatments and explore therapeutic advancements.
We curated this article using insights from our Synapse platform. Keep track of advancements in drug developments and clinical trials when you register for Synapse.
Psoriasis is a skin disorder that causes skin cells to multiply faster than normal. In the US alone, it affects nearly 7.5 million people. It occurs due to an overactive immune system and causes inflammation that impacts other organs and tissues in the body in addition to the skin.
One out of every three people with psoriasis may also develop psoriatic arthritis, a serious chronic disease where joints and tendons become inflamed and can lead to joint damage in later stages.
Currently, there is no cure for psoriasis, but there are methods for treating it. This article will dive into available treatments and explore therapeutic advancements.
Psoriasis Overview
As it stands, there are 30 approved treatments for psoriasis. These include steroid creams, shots, and topical ointments which aim to reduce itching and slow the production of skin cells. Unfortunately, these treatments are not without side effects. Some people experience skin irritation, swelling, and worsening of psoriasis. As such, industry leaders are looking for better and more efficacious treatment options.
The chart below illustrates key industry players, based on approved treatment options. These companies include Pfizer, GSK, and Eli Lilly. Right now, there are five drugs in phase III, two of which belong to Leo Pharma and Eli Lilly. One belongs to Novartis. Although these companies are the leaders there are many key players and small players in the space as well. Below we’ll dive into a specific example between Pfizer and Celltrion Inc., which showcases how these smaller players contribute to the psoriasis space and how larger manufacturers expand their treatment offerings. Keeping track of these deals and transactions is extremely important in any indication space.
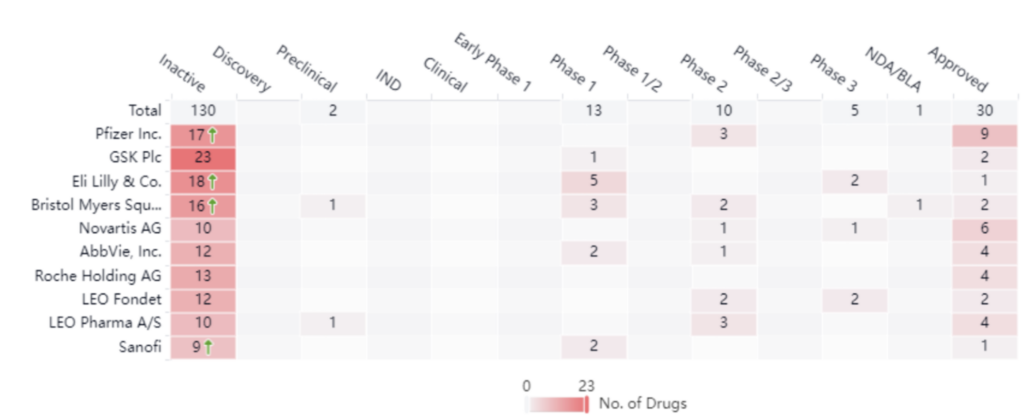
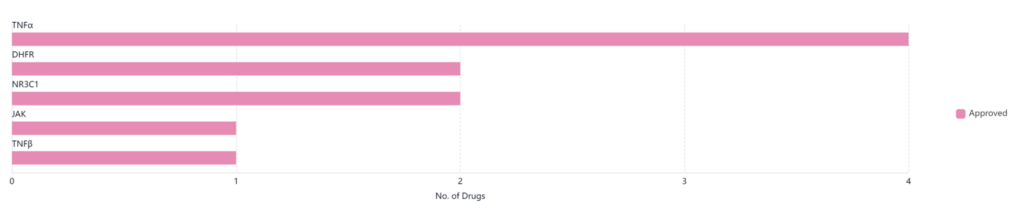
Pfizer leads with nine approved drugs in this space. Analyzing the graph below, we see that Pfizer treatments currently cover five different targets for treatment. Of these, four target TNF alpha, which is an inflammatory cytokine that’s responsible for many different signaling events in cells that lead to necrosis and apoptosis.
As an industry leader, it comes as no surprise that Pfizer diversifies its innovation strategy. In addition to maintaining its own research and development arm, Pfizer also has a robust licensing and acquisition strategy. For example, in 2016. Pfizer announced it would begin shipping infliximab-dyyb (INFLECTRA), which at the time had already been introduced into many other markets globally. Pfizer accomplished this by obtaining the exclusive commercialization rights in the US for the drug which belonged to Celltrion, Inc. Transactions such as this allow companies like Pfizer to add effective treatments to their portfolio.
Piclidenoson: A Psoriasis Breakthrough?
In recent news Can-Fite, a biotechnology company that focuses on advancing proprietary small molecule drugs (to treat diseases such as inflammatory, cancer, and liver disease) announced it’s going to submit registration plans to the FDA, and a Marketing Authorization Application with the EMA, for the drug Piclidenoson for treating moderate to severe psoriasis.
This comes after the company’s phase III trials of COMFORT TM showcased statistically significant improvement in patients who took the medication versus the placebo patients. As we analyze the study further, we find only one percent of patients on Piclidenoson or placebo had induced gastrointestinal adverse events whereas six percent of patients on Otezla (psoriasis medication made by AMGEN) had gastrointestinal adverse events. As such, the discontinuation of Otezla is higher for patients compared to patients treated with Piclidenoson. These results further quantify the excellent safety profile of Piclidenoson as a psoriasis treatment.
Potential Other Indications
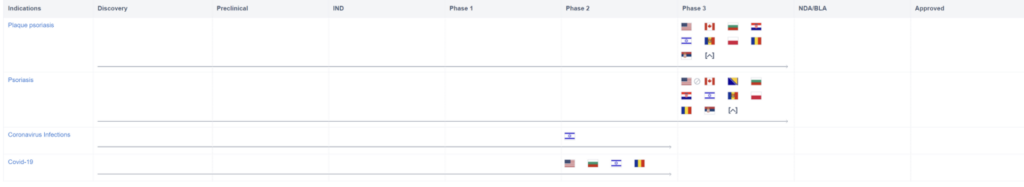
However, the use of Piclidenoson may not stop there. Take a look at the graph below. It shows that Piclidenoson is also being tested for other indications, most notably COVID-19. Phase II of the clinical study was recently completed on April 21, 2022.
In the cited clinical trials, Piclidenoson will be tested against a placebo in a population of hospitalized subjects with moderate or severe COVID-19. In this trial, Piclidenoson will be assessed through clinical, respiratory, and virologic parameters and the efficacy will also be assessed. Although Piclidenoson is making headway in the treatment of psoriasis, this could potentially lead to a whole new indication based on the repurposing of the drug and, of course, this is contingent on successful clinical trials of the treatment.
Conclusion
Research and development in the psoriasis space is constantly evolving. We can expect to see treatments from manufacturers continue to develop and grow. Like in any space we can keep an eye on drugs possibly being repurposed or repositioned for other indications, such as the example above with Piclidenoson.
If you’re interested in learning more about this space or keeping track of drug development and clinical trials, sign up for Synapse, our freemium product offering.
Author Bio

Nicholas Bevilacqua is the Product Marketing Manager For the Life Sciences Platforms at PatSnap. Nicholas holds an Honors Bachelors of Life Sciences from York University and an MBA in Strategic Marketing from the DeGroote School of Business at McMaster University. In his spare time, he enjoys cooking and outdoor activities.
Your recommended content
-

Patsnap Surpasses US$100 Million in Annual Recurring Revenue
Category: Article | Category: News/PR
Wednesday, June 12, 2024
Patsnap has reached a significant milestone of achieving $100M in Annual Recurring Revenue (ARR), marking an impressive 20% year-over-year growth in 2023. This milestone highlights the massive and meaningful value our platform brings to over 12,000 IP and R&D teams across 50 countries, driving efficiency, productivity, and collaboration.
-

Introducing Hiro, an AI assistant built for IP and R&D workflows
Category: AI advancements | Category: AI development | Category: AI-tools | Category: Article | Category: artificial intelligence
Tuesday, May 14, 2024
Powered by Patsnap’s industry-specific LLM, Hiro is designed to streamline IP and R&D workflows from ideation to product launch. With its robust AI capabilities, Hiro brings a new level of efficiency, precision, and security to tasks that were once time-consuming and labor-intensive.What sets Hiro apart is that it draws from our large language model that’s been trained on market-leading patent records, academic papers, and proprietary innovation data. This ensures we deliver more accurate and reliable results for every prompt.
-
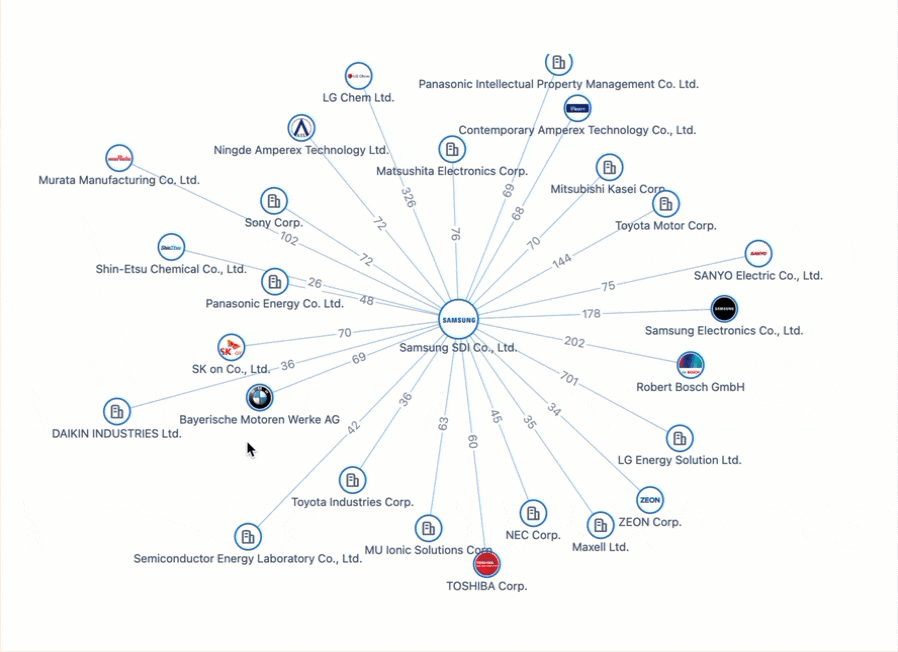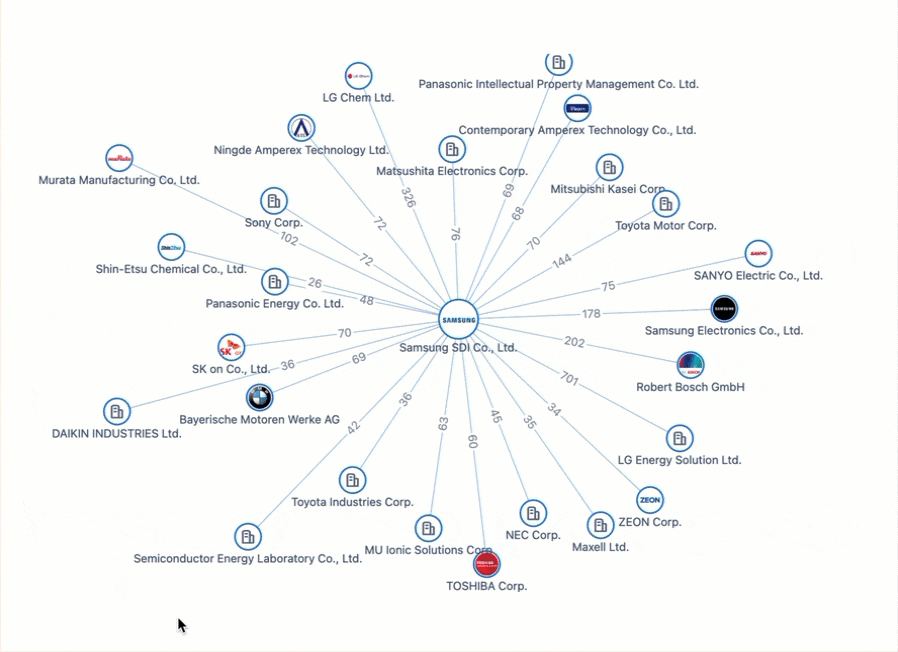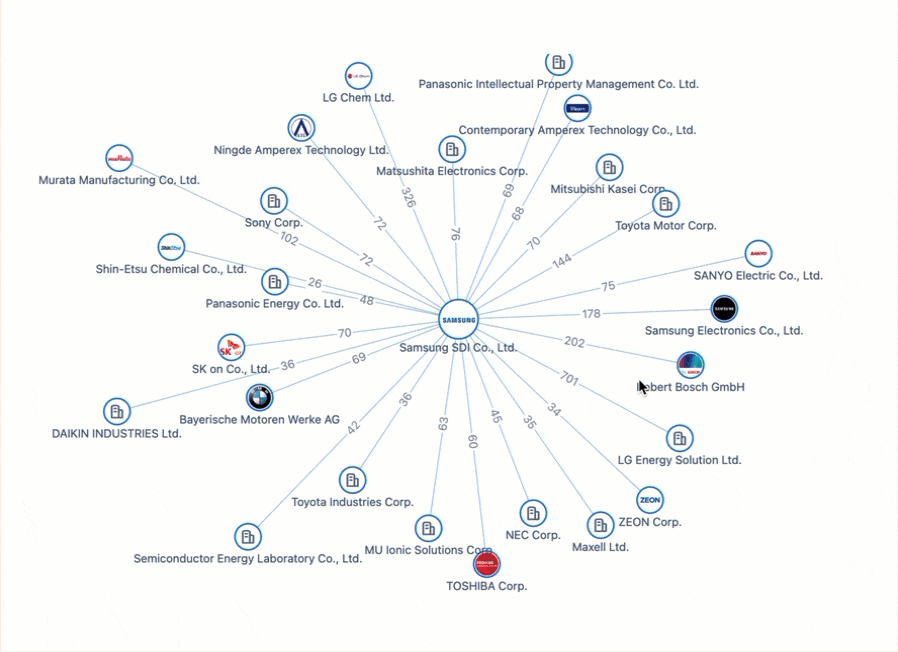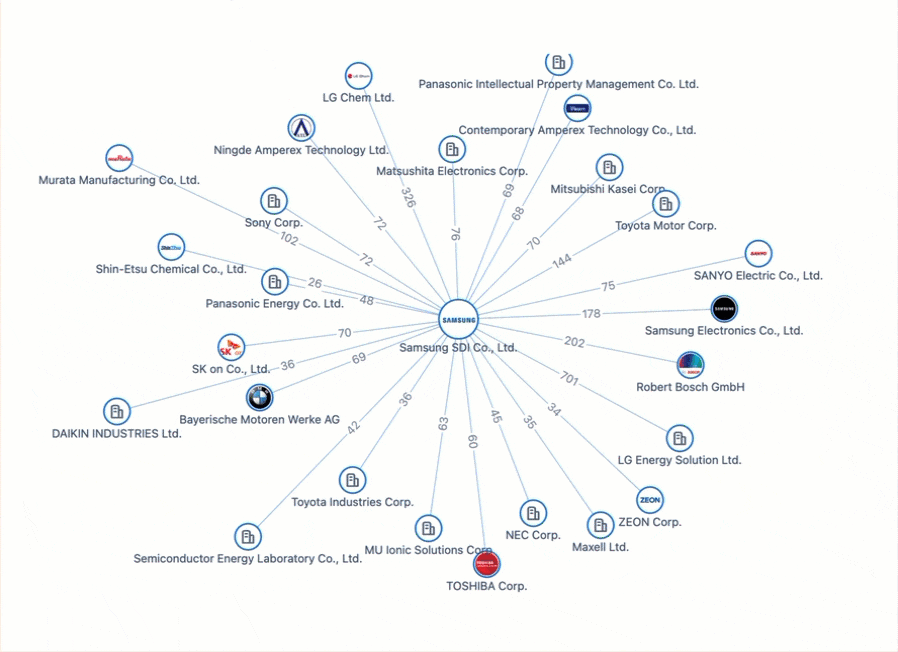
Powering the Future of Electric Vehicles: The Battle for Battery Innovation and Patents
Category: Article | Category: battery technology | Category: electric vehicle | Category: EV | Category: lithium ion | Category: lithium ion battery | Category: NEV | Category: new energy vehicles
Monday, April 22, 2024
In the ever-evolving landscape of innovation, the electric vehicle (EV) industry stands as a beacon of technological transformation. As we explore the patents propelling the EV revolution, Apple's venture serves as a poignant example of the challenges even industry giants face in this competitive arena. Join us on a journey through the global patent landscape, where the quest for superior power solutions unfolds, and where the true pioneers of the EV revolution are making their mark.